| References | |

|
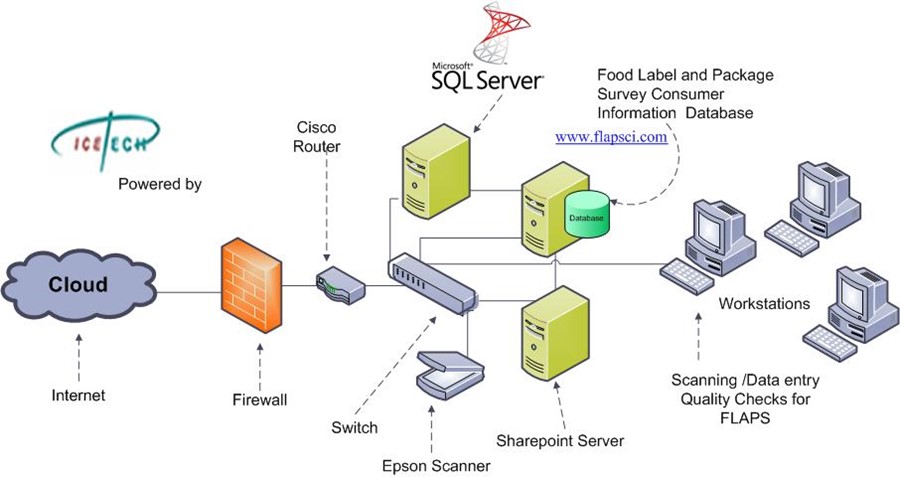
Label and Package Survey (FLAPS)Description of the Project: Icetech provided and deliver business, IT technical support and scientific research on the Nationwide Food UPC labeling codes and developed an enterprise web application and Oracle database solution for the FLAPS program that provides the most comprehensive overview of label information research on all food products in the U.S. Scope: Specifically, the FLAPS database will enable the Food and Drug Administration (FDA) to: (1) keep abreast of market responses to food labeling rules via changes in food package labels (e.g., trans fat labeling); (2) provided a sampling frame for studies that compare analytical nutrient values with the label values; (3) examine the types of ingredients that manufacturers are using; (4) review the serving size declarations on packaged foods; (5) determine the nutrient content claims and health claims that are being used on labels and the types of products that qualify for the claims; and (6) determine prevalence of food safety (e.g., refrigeration and pasteurization labeling) and other consumer information on food products. The FLAPS database will be able to use barcode technology and will have features with direct scanning functions. The database and site will be Section 508 Compliant.
1. Icetech designed a data entry form with the given fields. The form will be filled manually by the data entry operator for each product. 7. Product Admin Management
External Assessment of the CDER Quality Management System for the CMC Review ProcessDescription of the Project: Development of Office of Biotechnology Products Internal Quality Procedures (IQPs) for the FDA Chemistry Description of the Project: Development of Office of Biotechnology Products Internal Quality Procedures (IQPs) for the FDA Chemistry Icetech The overall project purpose is to implement and deploy the CDER Chemistry, Manufacturing, and Controls Quality Management System (CMC QMS), program specific internal quality procedures (IQPs) as recommended by the senior management team in OBP. This work covers all aspects of the CMC Review Process as defined by the Quality Management Plan (QMP), the program specific Quality Implementation Plan (QIP), and OPS Internal Quality Procedures. This includes activities by the contractor to coordinate, develop and implement program-level Internal Quality Procedures (IQPs) for OBP. The delivered contractor services shall contribute directly to how OBP and OPS meet quality system requirements as defined by the QMP in the following areas:
|